
TOP > 生物多様性センターの国際協力 > ESABII > Database > Threatened Mammal Species Database > Axis porcinus

Axis porcinus
Taxonomy (Name)
| Class | MAMMALIAIUCN | |
|---|---|---|
| Order | CETARTIODACTYLAIUCN | |
| Family | CERVIDAEIUCN | |
| Scientific Name | Axis porcinusIUCN | |
| Author | (Zimmermann, 1780)IUCN | |
| Synonyms | ||
| Common Name | Hog Deer, Indochinese Hog Deer, Thai Hog DeerIUCN | |
| Local name | Brunei Darussalam | |
| Cambodia | ||
| China | ||
| Indonesia | ||
| Japan | ||
| Lao PDR | ||
| Malaysia | ||
| Myanmar | ||
| Mongolia | ||
| Philippines | ||
| Singapore | ||
| Republic of Korea | ||
| Thailand | ?????????IUCN | |
| Vietnam | ||
Picture
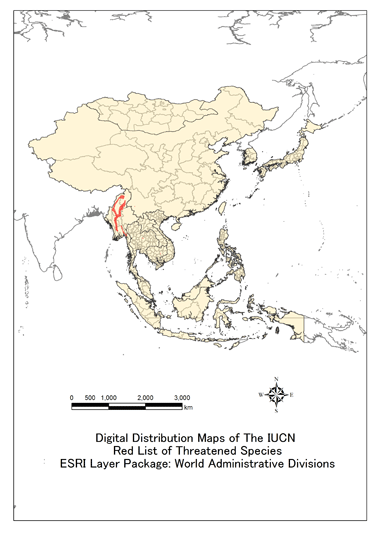
Distribution, Range
The Hog Deer historically occurred from Pakistan, throughout northern and northeastern India, including the Himalayan foothill zone, east across non-Sundaic Southeast Asia and, marginally, southern China (southern Yunnan province), but is now reduced to isolated populations within this range. It is almost extirpated from east of Myanmar. It is extinct in Thailand (where it has, however, been introduced) and almost certainly in Viet Nam and Lao PDR (Humphrey and Bain 1990; Duckworth et al. 1999; Tordoff et al. 2005; R.J. Timmins pers. comm. 2008). Very small numbers have been found recently in Bangladesh, in one small region of Cambodia (Khan 2004; Maxwell et al. 2007), and, reportedly, China (per B.P.L. Chan pers. comm. 2008); although details of the latter have not yet been traced, it is believed to involve a population of at most a few dozen, at one site. Hog Deer still probably occur in at least several areas of Myanmar (J.W. Duckworth in litt. 2008, from various sources), and localised populations survive in northern and northeastern India, Nepal, Bhutan (few recent data) and Pakistan (status uncertain) (Biswas and Mathur 2000; Biswas 2004). Hog Deer has been introduced into Sri Lanka, Australia (specifically the coastal regions of south and east Gippsland; Moore and Mayze 1990), and the United States (Texas, Florida, and Hawaii) (Grubb 2005) (but is not mapped in these last two).
Map

Country
| Brunei Darussalam | |
|---|---|
| Cambodia | |
| China | |
| Indonesia | |
| Japan | |
| Lao PDR | |
| Malaysia | |
| Myanmar | |
| Mongolia | |
| Philippines | |
| Singapore | |
| Republic of Korea | |
| Thailand | 再導入 |
| Vietnam |
Status
International Status
IUCN Red List Category
ENIUCN
Justification
The current most appropriate category for Hog Deer is Endangered A2bcd (past reduction of 50% or greater in three generations, taken here as about 21 years), through a combination of population trends across its range (see below). Reflecting the ever-increasing proportion of the total native population in well secured protected areas such as Kaziranga, despite an ongoing major reduction in occupancy and number of populations, future declines in the total number of animals (A3 and A4) are unlikely to be at rates in the next three generations sufficient for Endangered. However, without concerted action, within the next decade or two Hog Deer is likely to warrant again listing as Endangered through a different criterion, B2ac (c through episodic flood-caused mass mortality), reflecting the loss of all populations except a handful in protected areas with effective cohesive interventions against poaching and the wealth of other, habitat-related, threats facing Hog Deer. Currently the area of occupancy is far too high for listing as Endangered on this criterion, or even, probably, as Vulnerable.
During the mid and later decades of the twentieth century, the global population underwent rapid range-wide reductions, with the almost total loss of Hog Deer from South-east Asia (when it was widespread and numerous in much of Cambodia, southern Viet Nam, lowland Thailand and probably plains Lao PDR) and massive declines in the Terai Arc Landscape populations consequent upon the conquering of malaria and the influx of people to the area with wholesale habitat conversion and heavy hunting. Unquestionably at this time Hog Deer global population declines were at rates consistent with Endangered listing. By the mid 1980s (i.e., the start of the relevant period for a current A2 listing), populations (at least those large enough to influence the overall global decline rate) had gone from Thailand and Bangladesh and, almost certainly, from Lao PDR, Viet Nam and China. It is likely that many Hog Deer remained in Cambodia, where populations 2?3 decades ago may well have been in the thousands or even into the low tens of thousands, based on general perceptions of large-mammal abundance and relative wealth of suitable habitat (R.J. Timmins pers. comm. 2008), and Myanmar, where Hog Deer was regarded as a widespread and common species of no conservation concern in the mid 1980s (various people per J.W. Duckworth pers. comm. 2008). In the interim, the Cambodian population has collapsed. Although there is a dearth of information on current status in Myanmar, there is no reason to assume that such a trend is not also in train there, although it may potentially be somewhat behind Cambodia. In sum, the rate of population decline in the last 21 years in the region comprising Cambodia, Viet Nam, Lao PDR, Thailand, China and Bangladesh has exceeded 90%, a continuation of such declines from the 1950s onwards.
Populations in India and Nepal (and perhaps Pakistan and Bhutan; no recent information traced) have not declined at anything like such a rate in the last two decades. Declines in India are likely to have been more like 30?40% over the last 21 years, although this may be an underestimate (N.S. Kumar pers. comm. 2008); even in high-profile protected areas such as Corbett Tiger Reserve, Jaldapara Wildlife Sanctuary and Dudhwa Tiger Reserve populations are not secure. Declines have been at least 30% for Nepal (Hem Sagar Baral pers. comm. 2008).
The trend in the population in Myanmar and its proportionate contribution to the overall global trend over the last three generations is the chief uncertainty. It was surely numerically large enough to have some influence the global assessment of decline rate, given the abundance of prime habitat. Coupling the continued conversion of habitat to agriculture, the lack of protected areas specifically for the species (in contrast to those for Eld's Deer), the uneven success of Eld's Deer protected areas in conserving the species even with prioritised support and international collaboration, and the lack of awareness over the plight of grassland biodiversity in the country, as typified by current locations of protected areas, it is clear that whatever the surviving population of Hog Deer is in Myanmar, this is largely due to chance. In no other range state has chance resulted in the survival of large populations: there has been almost regional extinction in the countries to the east of Myanmar, and a fast retraction to reasonably well-secured protected areas in the countries to the west. The current reality for Myanmar probably lies somewhere between these two, and, thus, populations have probably declined by 40?60% or even more in the last three generations.
Combining these inferred regional decline rates, an overall global decline rate in the last 21 years of 50% is tenable. The Thai 'wild-living' population is re-introduced into artificially created habitats, and has not been used to influence the assessment; although it is within the species' native geographic range, it is likely that it is not the native subspecies. The Sri Lankan population, outside the native range, has not been used to influence the assessment (but seems to be declining anyway).
Hog Deer has not been previously listed as threatened. This was probably due in large part to a few large, high-density, South Asian populations that give the appearance of 'abundance', but these are exceptional to its true status, of extinction across most of its former range and within much of its present range. This is not fully appreciated even at the present time, despite the analysis of Biswas and Mathur (2000) and subsequent work by Biswas across one of the two main range states (India).
CITES
Appendix I (Axis porcinus annamiticusのみ)IUCN
CMS
National Status
| Country | Category | Reference |
|---|---|---|
| Brunei Darussalam | ||
| Cambodia | EN | Endangered Spacies in Cambodia(WWF) http://cambodia.panda.org/wwf_in_cambodia/endangered_species |
| China | Et | China Red Data book of Endangered Animals(1998) (Extinct, Extirpated, Endangered, Vulnerable, Rare, Interninate) |
| Indonesia | ||
| Japan | ||
| Korea | ||
| Lao PDR | CARL | Wildlife in Lao PDR, 1999 Status Report(IUCN, 1999) (At Risk in Lao, Conditionally At Risk, Little Known, not applicable, Potentially At Risk) |
| Malaysia | ||
| Mongolia | ||
| Myanmar | ||
| Philippines | ||
| Singapore | ||
| Thailand | EN (reintroduced) | Thailand Red Data: Mammals, Reptiles and Amphibians(Nabhitabhata and Chan-ard, 2005) |
| Vietnam |
Ecology Discription
Appearance
Habitat
Hog Deer have usually been reported from habitat consisting of wet or moist tall grasslands, often associated with medium- to large-sized rivers (Bhowmik et al. 1999; Biswas and Mathur 2000; Biswas 2004), and appears to reach its highest densities in floodplain grasslands (Seidensticker 1976; Dhungel and O'Gara 1991; Karanth and Nichols 2000; Odden et al. 2005). It avoids closed-canopy forest, but will use coastal grasslands (e.g. Peacock 1933). Johnsingh et al. (2004) considered Hog Deer to be an obligate grassland species in the Terai Arc Landscape of India, and studies in India and Nepal have show a preference for grasslands dominated by Imperata cylindrical (Biswas 2004 and references therein). Similar alluvial floodplain grassland seems to be used in Thailand and Indochina (Maxwell et al. 2007; Clark undated; R.J. Timmins pers. comm. 2006). In Bardia National Park, measured densities were much higher in the floodplain association than in the riverine association, and no Hog Deer were found in adjacent Sal Shorea robusta forest at all (Odden et al. 2005). The remnant population in Bangladesh is located in grassy, lightly wooded, hill country (Khan 2004). Similar habitats are used by Hog Deer in India, where they are seem to be marginal habitats supporting only low-density populations (Anwaruddin Choudhury pers. comm. 2006): they may historically have been primarily 'sink' populations. The reintroduced semi-wild populations in Thailand occupy a variety of habitats for which there is no evidence of use by wild populations in Thailand, Lao PDR, Viet Nam or Cambodia. One of the few detailed historical accounts of an abundant population in South-east Asia was from extensive tall floodplain grasslands in the Dong Nai catchment, Viet Nam (Clark undated).
Population size
Pakistan
Hog Deer are confined to isolated riverine grasslands along the Indus valley and its upper tributaries, mostly in the Indus River forest reserves of Sind Province, with small populations around the Indus mouth and to the north of Sukkur (Roberts 1977). The species was listed for the protected areas of Chashma Lake Wildlife Sanctuary, Head Islam/Chak Kotora Game Reserve (greatly reduced in number), Lal Suhanra National Park (reintroduced), Taunsa Barrage Wildlife Sanctuary, and possibly Rasool Barrage Wildlife Sanctuary (WCMC 1992), but no information more recent than Whale (1996) has been traced.
India
Hog Deer are found mainly in the terai grasslands along the Himalayan foothills and the flood-plains of the Rivers Ganges and Brahmaputra, from Punjab in the west to Arunachal Pradesh in the east (Tandon 1989; Johnsingh et al. 2004; Biswas 2004). Johnsingh et al. (2004), who made extensive surveys of the Terai Arc Landscape of India (for Tiger Panthera tigris and various prey species), considered that Dudhwa Tiger Reserve is excellent habitat for Hog Deer; that there was a reasonable population in Pilhibit Forest Division, particularly the Lagga Bagga forest block (now within a tiger reserve; B. Long pers. comm. 2008); that Kishanpur Wildife Sanctuary (203 km2), with one of the few remnants of terai habitat in India, supported considerable numbers, and that Katarniaghat Wildife Division also held Hog Deer. Assessments conducted specifically for Hog Deer by Biswas et al. (2002) (see also Biswas 2004) for India surmised that populations remained in 29?34 areas, with significant numbers only in the following: Arunachal Pradesh: 100?200 in D'ering, and 20?50 in Namdapha; Assam: 20?50 in Dibrusaikhowa, over 1,000 in Kaziranga (but see below), over 1,000 in Manas, 20?50 in Nameiri, 200?500 in Orang, 20?50 in Kobuchapori, 20?50 in Burachapori, and 20?50 in Sunai Rupa; Bihar: 100?200 in Valmiki; Uttrakand (= Uttaranchal): 200?500 in Corbett Tiger Reserve (but see below); Uttar Pradesh: 1,000?2,000 in Dudhwa, 20?50 in Katarniaghat, 100?200 in Kishanpur, and 20?50 in North Kheri Forest Division; and West Bengal: 100?200 in Gorumara and a population at Jaldapara. Their estimate for Pilhibit Forest Division was zero, contra Johnsingh et al. (2004). Biswas's study also considered that Hog Deer had been lost from 35 historically known localities, and suggested it was 'endangered', with citations given for alarming decline even prior to the mid 1970s (Biswas 2004). The figures were not derived from rigorous sampling and some will surely need revision. Particularly, it is certain that there are over 10,000 Hog Deer in Kaziranga, with a best estimate of 14,000?16,000, at ecological densities of 38.6 (with SE 3.45) animals per km2 (Karanth and Nicholls 2000; N.S. Kumar pers. comm. 2008); and in Corbett Tiger Reserve, only about 100 were reported in 2008 by Bivash Pandav (pers. comm. 2008) and while the actual number is unclear, densities are now very low, even in prime habitat (K.M. Chinnappa and Praveen per N.S. Kumar pers. comm. 2008), and a rapid decline is in place (Ajith Kumar pers. comm. 2008). Dudhwa Tiger Reserve also faces a suite of conservation problems meaning that population status of Hog Deer there is unlikely to be as healthy as in Kaziranga: poaching is rampant, and the creeper Tiliacora acuminate is proliferating across Hog Deer habitat (Kumar et al. 2002; Johnsingh et al. 2004; N.S. Kumar pers. comm. 2008). At Jaldapara Wildlife Sanctuary, where Biswas (1999) studied ecology of Hog Deer in the 1990s, sightings, and presumably populations, have now drastically declined (A.J.T. Johnsingh pers. comm. 2008). Hog Deer are now primarily restricted to protected areas (Anwaruddin Choudhury pers. comm. 2006), but south of Rajaji National Park there are a few isolated groups along the river (perhaps totalling about 100 animals); these are outside protected areas (Bivash Pandav pers. comm. 2008). All such populations outside protected areas are in very rapid decline (N.S. Kumar pers. comm. 2008). The presence of this species in Manipur and southern Assam (close to Manipur, Mizoram and Tripura) is based on records in Biswas et al. (2002); its current status there is unknown. All groups in these areas were estimated to be very small, and should not be assumed to persist.
Bangladesh
The Hog Deer have disappeared from the Sundarbans (Salter 1984) and has not been reported from the Sylhet District, in the northeast, since the 1970s (Khan 2004). After a long period with no records, an animal was trapped by local people in 2002 (Khan 2004). Further surveys suggested that a few Hog Deer remained in the Chitagong Hill Tracts of the southeast (Khan 2004; Md Anwarul Islam pers. comm. 2008). There is a small captive population derived from native animals (Md Anwarul Islam pers. comm. 2008). Within historic times, Hog Deer was never widespread in the country and pressure was on it from at least the 1970s (Md Anwarul Islam pers. comm. 2008).
Nepal
Hog Deer are locally abundant in the terai, but is now restricted largely, if not entirely, to protected areas (Pralad Yonzon pers. comm. 2006; Hem Sagar Baral pers. comm. 2008). Although populations may currently be relatively stable, declines in the past 20?30 years are likely to have exceeded 30% (Hem Sagar Baral pers. comm. 2008). Measured densities range from 0.1 per km2 in riverine forest, to 16.5 per km2 in savanna, and 35 per km2 in grassland flood-plains (Seidensticker 1976; Dhungel and O'Gara 1991). In Bardia National Park, densities of 77.3 animals per km2 in the floodplain association and 5.8 animals per km2 in the riverine association (and 0 in Sal forest) were measured (Odden et al. 2005). The species occurs in the following protected areas: Parsa Wildlife Reserve (with a very small population), Koshi Tappu Wildlife Reserve, Bardia National Park, Chitwan National Park, and Sukla Phanta Wildlife Reserve (Hem Sagar Baral pers. comm. 2008). Each of Suklaphanta, Bardia, and Chitwan supports hundreds of Hog Deer.
Bhutan
Hog Deer occur in the lowlands of southern Bhutan, but no information on current status has been traced. It occurs in at least two protected areas, Mochu Wildlife Reserve and Royal Manas National Park.
China
Hog Deer historically were recorded in China from the lowest valleys of Xishuangbanna in southern Yunnan, and has recently been stated to be extinct in the country (Ohtaishi and Gao 1990; Smith and Xie Yan 2008). Reportedly, however, an animal was reportedly found at Yongde Daxueshan National Nature Reserve, Yunnan (west of the Mekong), in 2007 (per B.P.L. Chan pers. comm. 2008).
Myanmar
Hog Deer have long been known in Myanmar (e.g. Evans 1902) and occurred throughout the country wherever there were grassy plains (Peacock 1933). Detailed information on Hog Deer's current range or population status in Myanmar has not been traced. It was already in major decline by the time Peacock (1933) wrote. It was listed only for three protected areas by WCMC (1992): Pidaung, Kahilu, and Hlawga Wildlife Reserves. Of these, the Hlawga population of many dozen (in 2007) is a semi-captive, managed herd: the entire area, of several square kilometers, is fenced; however, it may be 'native' in that the founders were reputedly from animals captured locally during the 1980s (Saw Htoo Tha Po verbally 2007 to J.W. Duckworth pers. comm. 2008). The Pidaung Wildlife Sanctuary was set aside in 1918 to protect an unusual mix of evergreen forest and savanna-like ecosystems (Milton et al. 1964), and Hog Deer used to be very numerous within it (Peacock 1933); at this time it was the only site in the country where Hog Deer received a measure of protection. Today, the presence of permanent human settlements, roads and railway lines, plantations of sugar cane, military camps, and permanent cultivation have completely altered large portions of the sanctuary, and an insignificant area of the original ecosystem remains (Rao et al. 2002). These land-uses are concentrated in the plains parts of the sanctuary, where Hog Deer used to live, and have converted so absolutely these areas that it is not obvious to someone driving along the road when the protected area is entered or exited. People and dogs are ubiquitous and it seems implausible that any Hog Deer could survive here (J.W. Duckworth pers. comm. 2008, based on visits in 2005?2006). There are, however, plausible hunters' and conservation staff reports from Hukaung Valley in Kachin state, and several government staff indicated in 2005?2007 that the species is still locally common in lowland Myanmar. However, firm evidence for widespread continued presence, rather than reporting of a status of some decades previously could not be found (J.W. Duckworth pers. comm. 2008). Pidaung was seen as a flagship protected area (it was one of the country's first), and its current status does not augur well for the few other protected areas which contain suitable Hog Deer habitat. Extensive camera-trapping for a Tiger survey found no evidence of the species (Lynam 2003), but this may simply have reflected the focus of the survey on closed evergreen forests (habitats unsuitable for Hog Deer), as various other species known to be common but not using closed evergreen forest were also camera-trapped rarely if at all (Than Zaw et al. 2008).
Thailand
The species was formerly abundant in the Chao Phraya Basin during the early 20th century, but had become extinct there by the mid-1960s (Humphrey and Bain 1990). There was formerly a huge export trade, for example 20,000 skins went out of the Gulf of Siam (it is not stated whether this was from Thailand, Cambodia, or both) in 1930?1931 (Dumas 1944). There was a plea for conservation attention to the species in Thailand in Lekagul and McNeely (1977), but this seems to have come too late. Animals reportedly from Myanmar have been used to build up numerous managed herds in captive and semi-wild conditions across the country. Such animals were introduced into Phu Khieo Wildlife Sanctuary and Propagation Station in northern Thailand in the early 1990s, at a higher elevation than is typical for the natural range of the species; this herd lives in open grassland around the edge of a reservoir (Anak Pattanavibool pers. comm. 2006; R. Steinmetz pers. comm. 2006). There is also an apparently large introduced population on the island of Ko Chang in the Gulf of Siam.
Lao PDR
Hog Deer are probably extinct in Lao PDR and its past status is difficult to assess: there are few, if any, specimens held in international collections from the country, and there were no records (except of old trophies) during extensive surveys across the country in the 1990s (Duckworth et al. 1999). However, Osgood (1932) specifically cautioned that it was much under-represented in zoological collections, despite it being locally common in Indochina. The extent to which this statement includes Lao PDR is unknown. All potentially suitable habitat patches are now small in size and are heavily used by local people, hunting pressure in Lao is high, and it is inconceivable in the great majority of areas that any Hog Deer could still persist (R.J. Timmins pers. comm. 2008).
Viet Nam
Hog Deer are thought to be extinct in Viet Nam, having previously occurred in some large populations in suitable habitat (e.g. Clark undated) and apparently having been widespread in the south according to reports in Ratajszczak (1991). This reflects the rapidity with which suitable habitat has been lost and the heavy hunting pressure on large mammals throughout much of Viet Nam (Tordoff et al. 2005; Timmins and Duckworth 2000). If the species still occurs in Viet Nam, it is most likely to be along the Kon river in Kon Cha Rang Nature Reserve, the western Srepok river lowlands of Dak Lak Province, the Sa Tay area of Kon Tum and Gia Lai Provinces, the Dong Nai river lowlands and Lam Dong Province (R.J. Timmins pers. comm. 2008). However, there is no recent evidence other than anecdotal reports, and a trophy which may be of Hog Deer from the Kon Cha Rang NR area. Other evidence, notably high hunting rates of ungulates in general, suggest that even very small numbers are unlikely to survive (Dang Huy Huynh 1986; Tordoff et al. 2005; R.J. Timmins pers. comm. 2006).
Cambodia
Hog Deer used to be abundant on the vast marshy plains of Cambodia, such as around Sre Ambel, in the province of Kratie, around Kompong Som, and around Kampot; in the drier regions of much of the north it was not common (Dumas 1944). Increased wildlife surveys from 1994 onwards failed to find Hog Deer anywhere in Cambodia until it was re-found in Kratie in 2006, following concerns it might already be extinct (Tordoff et al. 2005; Maxwell et al. 2007). A few animals, probably in the low dozens, remain scattered in small groups in remnant areas of floodplain grasslands and other vegetation in Kratie Province (Maxwell et al. 2007; Bezuijen et al. in prep.). Other such populations might remain, but this becomes progressively less likely as surveys cover more potential areas without finding the species, and as the country rapidly converts remaining semi-natural lowland river plains to agriculture (R.J. Timmins pers. comm. 2008).
Sri Lanka (introduced)
Hog Deer are restricted to largely cultivated landscapes within a 35 km2 area, between Ambalangoda and Indurawa on the southwest coast, and inland as far as Elpitiya (McCarthy and Dissanayake 1992, 1994). It does not occur in any protected area. Hog Deer was probably introduced to Sri Lanka by the Dutch or the Portugese in the 16th century, or possibly by a Sinhalese ruler in the pre-colonial period or the British later on; suggestions that it is native lack credibility because of the absence of records across central and southern India (Biswas and Mathur 2000).
Behavior
Where undisturbed, Hog Deer tend to be crepuscular, with significant day-time activity and some at night, especially in the hot and wet seasons (Dhungel and O'Gara 1991). In some areas it seems to have become more nocturnal and solitary (e.g. Cambodia; R.J. Timmins pers. comm. 2008), presumably through hunting pressure. The main social group is a female and fawn. When more Hog Deer are together, they do not form a strong "unit", fleeing when flushed in different directions rather than as one. In Chitwan, aggregations of up to 20 animals have been observed feeding on new shoots following fire (Dhungel and O'Gara 1991). In Kaziranga, aggregations of 40?80 animals are frequently seen on grazing grounds created by Great Indian Rhinoceros Rhinoceros unicornis and/or short grasslands near large water bodies (N.S. Kumar pers. comm. 2008, based on observations in 1996). Home ranges vary widely in size, but average about 5?70 ha, depending on how the range is defined (Dhungel and O'Gara 1991; Odden et al. 2005). In Chitwan, Hog Deer are essentially sedentary (Dhungel and O'Gara (1991), but in cultivated landscapes (Sri Lanka) movements are reported to be influenced by agricultural seasons (McCarthy and Dissanayake 1992). They move into higher-lying grasslands in response to monsoon flooding in India, Myanmar and presumably throughout their range (Peacock 1933; Q. Qureshi pers. comm. 1995).
Diet
Hog Deer is a primarily a grazer of young grasses, particularly Imperata cylindrica and Saccharum spp.; it also takes herbs, flowers, fruits, and browse (young leaves and shoots of shrubs) (Bhowmik et al. 1999; Dhungel and O'Gara 1991; Bisawas 2004; Wegge et al. 2006). It is much more a grazer and less a browser than is the Sambar Rusa unicolor. Introduced animals occur in scrub and cinnamon gardens in Sri Lanka, where they cause considerable damage to home crops (McCarthy and Dissanayake 1992).
Reproduction
The rut is during September?October in Nepal and India and (presumably based on captives) during September?February in China. 1?2 fawns are born during April?May in Nepal and during April?October in China. Gestation period is 220?230 days (Dhungel and O'Gara 1991; Sheng and Ohtaishi 1993). Fawns wean at six months, reaching sexual maturity at 8?12 months. The maximum recorded life span is 20 years.
Threat
Major Threat(s)
Hog Deer is threatened by habitat factors and by hunting, and much prime Hog Deer habitat had probably been converted well before the twentieth century (e.g. Peacock 1933). The balance between these threats and their current severity varies somewhat between 'South Asia' (here comprising India, Nepal, presumably Bhutan, and perhaps Pakistan) and 'South-east Asia' (here comprising China, Viet Nam, Lao PDR, Cambodia, Thailand, and Bangladesh). There is insufficient information to judge for Myanmar into which group it would fit, reflecting current threats and resultant Hog Deer status. Sri Lanka is outside the native range so not considered in detail here.
In the Mekong countries of Lao PDR, Cambodia, Thailand, Viet Nam, and also probably China and Bangladesh, hunting has been and remains the primary direct threat to the species. In addition to local consumption of meat, the main factor presently driving such hunting is the thriving and probably increasing national, regional and East Asian markets trade in bushmeat, abetted by markets for traditional medicinal products derived from deer species and for trophy antlers (Tordoff et al. 2005; Maxwell et al. 2007). It is also possible that hunting could be stimulated by demand for captive animals, especially from zoos and menageries in Thailand and Cambodia (R.J. Timmins pers. comm. 2008). The species is apparently easy to hunt compared with other sympatric deer (e.g. Peacock 1933, Dumas 1944), and fawns are likely to be very vulnerable to dogs which almost always accompany human parties during excursions, however, brief, outside farm and residential land, even if these are not for hunting (Tordoff et al. 2005; R.J. Timmins pers. comm. 2008). Hunting itself has been greatly augmented by habitat loss: the vast majority of tall floodplain grasslands have been lost in the region due to the suitability of such habitat for human settlement and agriculture, and only very small patches remain. This huge extent of habitat loss probably occurred largely after Hog Deer had been hunted down to negligible numbers (R.J. Timmins pers. comm. 2008). Given these threats, which are ongoing, Hog Deer is one of the most threatened large mammals in Indochina (Timmins and Duckworth 2000; Tordoff et al. 2005; R.J. Timmins pers. comm. 2008). The most significant challenge to conserving the species there is the uncertainty involved with long term protected area-based conservation management. The area in Cambodia from which deer are known has been proposed as a protected area, but it remains to be seen if deer could be protected within the area.
Even in South Asia, where many protected areas are effective in conserving populations of large ungulates, Hog Deer declined precipitously in the twentieth century through habitat loss and hunting. It is still in decline even within some protected areas (e.g. Bhowmik 2002; see below). In India, habitat loss to cultivation has greatly reduced the area available to Hog Deer, and has continued to do so outside protected areas even in recent decades (N.S. Kumar pers. comm. 2008). Johnsingh et al. (2004) stated that high levels of malaria had previously discouraged settlement, agriculture, and thus habitat conversion in the Terai Arc Landscape, and that the conquering of malaria has made the Indian side of the Terai Arc Landscape "one of the most densely populated regions of [India, with a] population growing at a much higher rate than the rest of [India]. Most of the fertile terai plains have been taken over by agriculture". Johnsingh et al. (2004) stated that controlling disturbance to Lagga Bagga forest block in Pilibhit Forest Division (now within a tiger reserve; B. Long pers. comm. 2008) is a huge challenge: many people rely on it for firewood, thatch grass and fodder, and that intensive protection and participatory management with these people are required to restore the area. Such a threat profile is typical of areas supporting Hog Deer. Outside protected areas, some Hog Deer remain in woodland areas where densities are very low, and although animals do appear to breed in such situations, it is unclear in the absence of adjacent high-density river plains populations how viable such woodland populations will be in the long term (A. Choudhury pers. comm. 2006). Protected areas are encroached for cultivation and even more so for livestock grazing, which is surely increasing pressure on Hog Deer along with many other species. As a grazing species, Hog Deer numbers are likely to be severely depressed by competition with domestic livestock (by analogy with other wild ruminants, as detailed by Madhusudan 2004), although no data were traced on this. Domestic stock are ubiquitous in all areas outside the protected area network and even occur in many within it, specifically at least: Chitwan; Orang; Jaldapara; and Gorumara (Johnsingh et al. 2004; R.H. Emslie pers. comm.; B.N. Talukdar pers. comm. S.S. Bist pers. comm.).
Most populations in India are under severe threat from hunting (N.S. Kumar pers. comm. 2008), although the largest population of the species, in Kaziranga, has been secured from this threat for many years. Poaching occurs in protected areas throughout the Indian range, especially in north Bengal, and can be associated with other human uses of protected areas perceived to be more legitimate, such as grazing camps (Biswas 2004). Many old skulls of Hog Deer were found across Arunachal Pradesh and Assam during the survey of Biswas et al. (2002). On average 2?3 skulls were recorded from most houses in villages randomly visited during the survey in Arunachal Pradesh. Regularly, shots were heard and machans (tree-houses made for poaching) were found during the surveys in Nameri National Park, Sonai-Rupai Wildlife Sanctuary and Namdapha Tiger Reserve. According to questionnaire, 52% of responses reported high incidence of Hog Deer hunting in neighbouring areas. Hunting was reported as the primary reason for the sudden decline of the Hog Deer population from north Bengal since 1877. Biswas et al. (2002) detailed evidence of Hog Deer hunting during the survey and past records from the district of Cooch Behar (West Bengal) back to the 18th century. Even today, even in high-profile areas such a Corbett Tiger Reserve and Rajaji National Park, there are still instances of deer poaching, and poaching has seriously depleted abundance of large mammals in most of the Terai Arc Landscape. Some poaching is driven by a desire to seek adventure, the thrill of shikar and wild meat, some by crushing poverty of daily wage labourers and others, some to take advantage of the business opportunity of supplying a lucrative trade. Poaching is done with dogs, snares, spears and guns (Johnsingh et al. 2004). Outside protected areas in India, enforcement of anti-hunting regulations is patchy and, unless there is effective specific intervention, all Hog Deer populations in such areas will become extirpated in the near future from hunting (A. Choudhury pers. comm. 2006; N.S. Kumar pers. comm. 2008). Hog Deer avidly eats rice and so in the past was "destroyed ruthlessly on such grounds" (Peacock 1933). It seems unlikely that killing Hog Deer solely for reduction of crop damage is nowadays a reason for population decline, although it may be a convenient excuse to kill deer. Most serious is that the predilection of Hog Deer to eat rice is likely to being them into closer contact with people and therefore increase easy opportunities for hunting. In Kratie, Cambodia, Hog Deer occur near, and seasonally forage in, rice fields, especially in the early rainy season; people set traps for wild pigs Sus sp(p). (which are more abundant than Hog Deer), and the traps sometimes kill Hog Deer. The local people now are reportedly refraining from using indiscriminate traps in the Hog Deer area, but such cooperation is unlikely to continue forever, because crop predation by pigs is really a problem (A. Maxwell pers. comm. 2008).
In today's highly fragmented populations which have limited dispersal possibility, flooding may take heavy tolls, especially in the Brahmaputra flood plain (Q. Qureshi pers. comm. 1995). In Kaziranga National Park, where the largest population of this species is found, flooding is likely to be a major threat: in 1998 the flood reduced the population to less than half its pre-flood number (A. Choudhury pers. comm. 2006). The situation is similar in Nepal where Hog Deer appears to be especially vulnerable because of the short-grass habitats it usually uses during flood season, and in protected area like Koshi Tappu, flooding probably causes wide fluctuations in population numbers (Hem Sagar Baral pers. comm. 2008). Hog Deer populations appear to rebound quickly even from heavy losses, but the fragmentation and increasing isolation of populations into small areas, sometimes with no linkage to suitable higher ground, makes periodic losses from flooding a high risk for future site-level extinctions. Flooding is also a danger because, except in areas of effective anti-poaching activity, by marooning Hog Deer on little patches of high ground it makes them more easy to hunt (Peacock 1933).
A further threat also reflects the colossal habitat fragmentation in recent centuries. Prime Hog Deer habitats are naturally dynamic, and on a living floodplain new areas of grasslands are constantly created through natural processes to replace those changing through natural succession to woody vegetation; creation occurs through traumatic change of floodplain stream and land layout during spates (Dinerstein 1979; Lehmkuhl 1994; Peet et al. 1999). This is incompatible with farming and other human land-uses, which seek a predictable landscape. Hence, within Hog Deer range, there are very few active natural floodplains in which pre-exploitation processes shape the landscape in this way. Thus, the grasslands which are the prime habitat of Hog Deer generally require active management. In some protected areas grasslands are being lost by succession of wooded habitats, for example Chitwan (A. Choudhury pers. comm. 2006) and Bardia (Odden et al. 2005). By contrast, annual burning in Kaziranga, while maintaining a grass habitat, has rapidly changed the grassland structure and composition and how this change affects Hog Deer populations is not known (S. Deb Roy pers. comm. 1996). In Nepal there is also succession of different grassland types and in particular Imperata cylindrica grasslands, which Hog Deer appears to favour at certain times of the year (Biswas 2004), are being replaced by larger cane grasses (Hem Sagar Baral pers. comm. 2008). Locally, conservation management of grasslands to benefit rhinoceroses, for instance in Jaldapara, might negatively affect Hog Deer (Biswas 2004 and references within).
The invasive creeper Mikania sp. threatens Hog Deer's favoured grasslands both within and outside protected areas, and after flooding and poaching is possibly the next most serious threat to the species in Nepal (Hem Sagar Baral pers. comm. 2008). In combination with invasion by Chromolaena odorata (=Eupatorium odoratum), heavy livestock grazing pressure and invasion of some grasslands by Acacia catechu and Dalbergia sissou, the grassland area in Chitwan has been reduced from 20% to 4.7% of the national park (R.H. Emslie pers. comm.). Mikania sp. Is also carpeting large areas of non-forest habitats in the Hukaung Valley, Myanmar (J.W. Duckworth pers. comm. 2008). Lantana camara is also displacing native plants widely, and in Dudhwa Tiger Reserve, the creeper Tiliacora acuminata is proliferating across Hog Deer habitat (Kumar et al. 2002; Johnsingh et al. 2004; N.S. Kumar pers. comm. 2008). In Assam an invasive Mimosa sp. threatens the habitat in sites like Kaziranga and Orang National Parks, whereas in Manas National Park an invasive Lea sp. has become a major threat to the grassland habitat of the hog deer (B. Talukdar pers. comm. 2008).
Floodplain grasslands are strongly affected in their pace of natural succession by water flows and levels, and seasonal patterns in them. Hydropower development changes these parameters and thus threatens habitat, including a project under consideration across the Mekong which would affect the sole known population of A. p. annamiticus (that in Cambodia), and another across the Karnali river which would modify habitats in Bardia, Nepal (Odden et al. 2005). There have been proposals to dam the Brahmaputra River in Arunachal Pradesh, and should this happen, this could very negatively affect the habitat quality and Hog Deer carrying capacity of major parks like Kaziranga in future (by preventing or reducing the pulse of nutrients brought in by regular large floods, as well as changes in water levels and flows). In Jaldapara Sanctuary, the River Torsa no longer overflows as a result of massive flood-control structures. As a result the water table in the sanctuary is receding and the natural water-bodies and wallow-pools are slowly drying up (S.S. Bist pers. comm.).
Disease epidemics spreading from domestic livestock presumably pose a threat, especially given the close overlap of Hog Deer and domestic livestock in South Asia, the high densities especially of the latter, and the small and localised nature of Hog Deer populations.
In the introduced range, in Sri Lanka the continued survival of the introduced populations depends on controlling hunting and maintaining traditional agricultural land use practices. The land is too intensively cultivated for the establishment of protected areas within the range of the species. Threats in the rest of the introduced range are not reviewed here.
Conservation and Measurement
International
The Hog Deer is fully protected in Bangladesh, India, and probably most or all other range states. One subspecies is listed on CITES Appendix I as Axis porcinus annamiticus.
National
Wetlands, especially floodplain grasslands, have traditionally been ignored by the protected area systems and other conservation initiatives of Lao PDR, Viet Nam, and Cambodia (Tordoff et al. 2005; R.J. Timmins pers. comm. 2006). Most of the area supporting the single known population in Cambodia has been proposed for protected area status, but the situation is complicated by the inclusion of agricultural lands and traditional use areas of local people, and the high human population immediately nearby (Maxwell et al. 2007; Bezuijen et al. in prep.). As soon as the population was found, a small conservation programme with external funding from WWF was started to support a small number of forestry rangers and local guardians to carry out patrolling and community outreach activities, in addition to basic research. To ensure protection and survival of animals however, consideration should be given as to whether it would be useful for part of the area to be strategically fenced to help deter poaching, agricultural encroachment and predation by dogs; but this would need to consider seasonal movements. There are few areas in Viet Nam where suitable habitat is thought to remain, but if reasonable extents of alluvial grassland survive in the following areas surveys for Hog Deer may be warranted: Cat Tien National Park, the Kon river in the Kon Cha Rang Nature Reserve area, and the Sa Tay area of Kon Tum and Gia Lai Provinces.
It is not certain what conservation measures are in place (if any) for the rediscovered population in Bangladesh.
Hog Deer in Myanmar has not been surveyed recently, but probably exists mostly in unprotected plains grassland areas which, as in the Mekong countries, are conventionally seen as of low conservation value (Peacock 1933; J.W. Duckworth pers. comm. 2006). The Hukaung valley riverine grasslands had been considered, therefore, suitable as areas for agricultural conversion, but there are proposals for some of them to be included in a forthcoming expansion to the Hukaung Wildlife Sanctuary (J.W. Duckworth and Than Zaw pers. comm. 2006). A clarification of the status of Hog Deer in Myanmar is urgent: there may yet remain areas supporting animals which have high conservation potential if they do not disappear before being known to conservation. This lack of conservation profile is the chief challenge to Hog Deer in Myanmar at the moment, as it was in Peacock's (1933) day: "the butchery of these little deer and the failure to provide more than one small sanctuary for their preservation is certainly one of the weak spots in Myanmar game legislation...the existing legislation is singularly ineffective in preventing their wholesale destruction. Moreover, there are few forest reserves which are suitable for the Hog Deer, and the legislation concerned with such forests is little more than a gesture". If not met head-on, there are no grounds to assume that Hog Deer national conservation status will not replicate that shown by all range states to the east (major population collapse), if indeed it has not already done so.
Most Hog Deer populations remaining in India, Nepal, and Bhutan are in protected areas where animals are at least somewhat, and in some cases well, protected from poaching (Biswas et al. 2002; Biswas 2004; Johnsingh et al. 2004; Pralad Yonzon pers. comm. 2006; Hem Sagar Baral pers. comm. 2008; A. Choudhury pers. comm. 2006; N.S. Kumar pers. comm. 2008). Wildlife protection laws in India have also helped in retaining the specialized habitat of Hog Deer, arresting habitat loss and habitat conversion in some protected areas. Johnsingh et al. (2004) found a strong correlation with Hog Deer populations and protected areas in the Terai Arc Landscape of India (similar to that for Barasingha Rucervus duvaucelii), in contrast to other deer which occur relatively widely outside protected areas. Some protected areas are not big enough to allow adequate ranging during heavy flood periods, and thus prevent heavy mortality during such periods, and all suffer from being too small to be allowed unfettered habitat succession, because new grasslands are not now being created by natural processes elsewhere (see Habitat and ecology). In India and Nepal, Hog Deer has benefited from conservation measures taken for Great Indian Rhinoceros and Barasingha in the same wet grassland habitats (Q. Qureshi pers. comm. 1995; Biswas 2004). However, grassland management for rhinoceros may in some cases be deleterious to Hog Deer (Biswas 2004). Investigation of Kaziranga National Park during 2008 will clarify the conservation status of Hog Deer there (N.S. Kumar pers. comm. 2008).
In South Asia there is an urgent need to update the status review of Biswas and Mathur (2000), even though some large Hog Deer populations inhabit some of the best-secured protected areas in the world. Even assuming that these protected areas continue to receive sufficient material and political support, such a review is necessary because all populations face uncertain futures from a multitude of factors, some of which originate outside protected areas and beyond the zone of influence of protected area managers. The review needs to determine roughly the numbers remaining and the locations of all sizeable or potentially sizeable populations, and especially for each make assessments of current and likely future conservation status and population trends, bearing in mind threats from poaching, agricultural encroachment, livestock grazing, natural habitat succession, perverse habitat management, invasive plant species, the risk of disease epidemics from domestic livestock, changes to water levels and flows (e.g. from upstream dams), further fragmentation of populations and habitats, episodic mortality through flooding and abiotic risks such as insurgency (which can collapse protected area management, although it does not always do so). Confinement of populations to small habitat patches isolated from other such patches raises the intrinsic threat of subpopulation loss significantly when considering the wide gamut of threats facing such populations (Biswas and Singh 2002; Biswas 2004), and in some sites challenges may be so pervasive that long-term retention of Hog Deer populations may be so difficult as to be an inefficient use of resources. An integrated South Asia-wide conservation plan may be the best route to direct resources to a representative set of areas where long-term persistence of large Hog Deer populations can be assured provided sufficient resources are mobilised and deployed in the appropriate way.
By contrast, in Southeast Asia, any population found east of central Thailand (i.e. within the historical range of A. p. annamiticus, the western limit of which is not clear) warrants immediate attention. It is plausible that the recently found small and fragile population in Cambodia is the last stock of this taxon. The isolated small populations in Bangladesh and China, although presumably (particularly in Bangladesh) of the nominate race, warrant priority protection in maintaining the ancestral geographic and habitat range of Hog Deer. Species-specific intervention in Myanmar should probably, given the great challenges in the country (and, specifically, the uneven success to date with Eld's Deer Rucervus eldii conservation), be fitted within larger initiatives such as conservation of the Hukaung Valley, probably the largest floodplain in tropical Asia retaining largely natural ecological processes of stream geography and habitat dynamics.
In Sri Lanka, where the species is restricted to privately-owned gardens, its survival depends on the goodwill of the landowners. The conservation value of this introduced population needs re-evaluation, considering approximate population size, recent population trend, ecological integrity of the population and any genetic uniqueness, balanced against the likely cost of conservation interventions to secure the population's future. Needs of other introduced populations are not considered here.
There is a large total of animals in zoos of tropical Asia. Although at least one Mekong animal appeared in a zoo recently (in Cambodia in the mid 1990s; C.M. Poole pers. comm. 1998), so far as is known, all captive herds are derived from populations from Myanmar and further west. Reintroduction of animals in the range of the nominate, should it be needed, thus has many options. There is no captive buffer for A. p. annamiticus.
Conservation law
| Country | Status | Reference |
|---|---|---|
| Brunei Darussalam | ||
| Cambodia | ||
| China | ||
| Indonesia | ||
| Japan | ||
| Korea | ||
| Lao PDR | ||
| Malaysia | ||
| Mongolia | ||
| Myanmar | Seasonal Protected Animals | Protection of Wildlife, Wild Plants and Conservation of Natural Areas Act 15(A), Forest Department Notification No. 583/94 |
| Philippines | ||
| Singapore | ||
| Thailand | ? | Wildlife Reservation and Protection Act B. E. 2535(1992) |
| Vietnam | Group I: Prohibiting Exploitation and Use for Commercial Purposes | The Government Decree 32/2006/ND-CP, Dated 30th March 2006 on Management of Endangered, Precious and Rare Species of Wild Plants and Animals |
Protected Area
Other Coservation Projects
Citation
Bezuijen, M. R., Timmins, R. and Seng, T. (eds). In press. Biological surveys of the Mekong River between Kratie and Stung Treng Towns, northeast Cambodia, 2006-2007. WWF Greater Mekong Programme, Cambodia Fisheries Administration and Cambodia Forestry Administration, Phnom Penh.
Bhowmik, M. K. 2002. The causes of decline of hog deer (Axis porcinus) in protected areas of Himalayan West Bengal. Zoos' Print Journal 17(8): 858-860.
Bhowmik, M. K., Chakraborty, T. and Raha, A. K. 1999. The habitat and food habits of hog deer (Axis porcinus) in protected areas of sub-Himalayan West Bengal. Tiger Paper 26(2): 25-27.
Biswas, T. 1999. Habitat utilization by Hog Deer (Axis Porcinus) in relation to other sympatric species at Jaldapara Wildlife Sanctuary. Saurashtra University.
Biswas, T. 2004. Hog Deer (Axis porcinus Zimmerman, 1780). ENVIS Bulletin (Wildlife Institute of India, Dehra Dun) 7: 61?78.
Biswas, T. and Mathur, V. B. 2000. A review of the present conservation scenario of Hog deer (Axis porcinus) in its native range. Indian Forester 126(10): 1068-1084.
Biswas, T. and Singh, S. 2002. Status and distribution of Hog Deer (Axis porcinus) in the duars - analyzing a changing landscape. Forestry and Ecology Division, Indian Institute of Remote Sensing.
Biswas, T, Mathur, V. B. and Sawarkar, V. B. 2002. Status of Hog Deer (Axis porcinus) in India. Wildlife Institute of India, Dehradun, India.
Clark, J. C. 1936. My Indo-China hunt [typewritten unbound account of the 1936 Fleischmann-Clark, American Museum, Indo-China Expedition]. New York, USA.
Dang, H. H., Dao, D. V. T., Cao, V. S., Pham, T. A. and Hoang, M. K. 1994. Checklist of mammals in Vietnam. Publishing House, Hanoi, Vietnam.
Dhungel, S. K. and O'Gara, B. W. 1991. Ecology of the hog deer in Royal Chitwan National Park, Nepal. Wildlife Monographs 119: 1-40.
Dinerstein, E. 1979. An ecological survey of the Royal Karnali Bardia Wildlife Reserve, Nepal 2. Habitat and animal interactions. Biological Conservation 16(4): 265-300.
Duckworth, J. W., Salter, R. E. and Khounbline, K. 1999. Wildlife in Lao PDR: 1999 Status Report. IUCN, Vientiane, Laos.
Dumas, C. 1944. La faune sauvage du Cambodge. Editions Aymonier, Phnom Penh.
Evans, G. H. 1902. Notes on the Hog Deer in Burma. Journal of the Bombay Natural History Society 14: 310-315.
Grubb, P. 2005. Artiodactyla. In: D. E. Wilson and D. M. Reeder (eds), Mammal Species of the World. A Taxonomic and Geographic Reference (3rd ed), pp. 637-722. Johns Hopkins University Press, Baltimore, USA.
Humphrey, S. R. and Bain, J. R. 1990. Endangered mammals of Thailand. Sandhill Crane Press Incorporated, Gainsville, Florida, USA.
Johnsingh, A. J. T., Qureshi, Q., Goyal, S. P., Rawat, G. S., Ramesh, K., David, A., Rajapandian, K. and Prasad, S. 2004. Conservation Status of Tiger and Associated Species in the Terai Arc Landscape, India.
Karanth, K. U. and Nichols, J. D. 2000. Ecological status and conservation of tigers in India. Centre for Wildlife Studies, Bangalore, India.
Khan, M. M. H. 2004. A report on the existence of wild Hog Deers in Bangladesh. Bangladesh Journal of Zoology 32: 111?112.
Kumar, H., Mathur, P. K., Lehmkuhl, J. F., Khati, D. S., Dr., R. and Longwah, W. 2002. Management of forests for biological diversity and forests productivity: a new perspective, vol. VI: Terai Conservation Area (TCA). WII-USDA Forest Service Collaborative Project Report, Wildlife Institute of India, Dehra Dun, India.
Lehmkuhl, J. F. 1994. A classification of subtropical riverine grassland and forest in Chitwan National Park, Nepal. Vegetatio 111: 29-43.
Lekagul, B. and Mcneely, J. A. 1988. Mammals of Thailand. White Lotus Press, Bangkok, Thailand.
Lynam, A. J. 2003. A National Tiger Action Plan for the Union of Myanmar. Myanmar Forest Department, Ministry of Forestry, Yangon, Myanmar.
Madhusudan, M. D. 2004. Recovery of wild large herbivores following livestock decline in a tropical Indian wildlife reserve. Journal of Applied Ecology 41: 858?869.
Maxwell, A., Chea Nareth, Duong Kong, Timmins, R. and Duckworth, J. W. 2007. Hog Deer (Axis porcinus) confirmed in the wild in eastern Cambodia. Natural History Bulletin of the Siam Society 54: 227?237.
McCarthy, A. J. and Dissanayake, S. 1992. Status of the hog deer (Axis porcinus) in Sri Lanka. Report to Department of Wildlife Conservation, Colombo. Department of Wildlife Conservation.
McCarthy, A. J., Dissanayake, S. B. 1994. Status of the hog deer in Sri Lanka. Oryx 28(1): 62-66.
Meijaard, I. and Groves, C. P. 2004. Morphometrical relationships between South-east Asian deer (Cervidae, tribe Cervini): evolutionary and biogeographic implications. Journal of Zoology (London) 263: 179-196.
Milton, O., Estes, R. D. and Kimlai, H. Z. 1964. Burma Wildlife Survey Report on the Pidaung Wildlife Sanctuary. Burmese Forester 14: 54-68.
Moe, S. R. 1995. Distribution and Movement Pattern of Deer in Response to Food Quality and Manipulation of Grassy Habitat: A Case Study With Emphasis On Axis Deer (Axis axis) in Lowland Nepal. Ph.D. Thesis, Norges Landbrukshogskole.
Moore, G. and Mayze, R. 1990. The hog deer. Australian Deer Research Foundation, Croydon, Australia.
Morten Odden and Per Wegge. 2006. Predicting spacing behavior and mating systems of solitary cervids: A study of hog deer and Indian muntjac. Department of Ecology and Natural Resource Management.
Odden, M., Wegge, P. and Storaas, T. 2005. Hog deer Axis porcinus need threatened tallgrass floodplains: a study of habitat selection in lowland Nepal. Animal Conservation 8: 99?104.
Ohtaishi, N. and Gao, Y. T. 1990. A review of the distribution of all species of deer (Tragulidae, Moschidae and Cervidae) in China. Mammal Review 20(2-3): 125-144.
Osgood, W. H. 1932. Mammals of the Kelley-Roosevelts and Delacour Asiatic expeditions. Field Museum of Natural History, Zoology Series 18(10): 193-339.
Peacock, E. H. 1933. A Game-Book for Burma and Adjoining Territories. Witherby, London, UK.
Peet, N. B., Watkinson, A. R., Bell, D. J. and Kattel, B. J. 1999. Plant diversity in the threatened subtropical grasslands of Nepal. Biological Conservation 88: 193-206.
Pitra, C., Fickel, J., Meijaard, E. and Groves, C. 2004. Evolution and phylogeny of old world deer. Molecular Phylogenetics and Evolution 33: 880-895.
Rao, M., Rabinowitz, A. R. and Khaing, S. T. 2002. Status review of the protected-area system in Myanmar, with recommendations for conservation planning. Conservation Biology 16(2): 360.
Ratajszczak, R. 1991. The distribution and status of deer in Vietnam.
Roberts, T. J. 1977. The Mammals of Pakistan. Ernest Benn, London, UK.
Salter, R. E. 1984. Integrated development of the Sundarbans, Bangladesh: status and utilization of wildlife. FAO, Rome, Italy.
Seidensticker, J. 1976. Ungulate populations in Chitawan valley, Nepal. Biological Conservation 10: 183-210.
Sheng, H. I. and Ohtaishi, N. 1993. The status of deer in China. In: N. Ohtaishi and H. I. Sheng (eds), Deer of China: Biology and Management, pp. 8. Elsevier, Oxford, UK.
Smith, A. and Xie, Y. 2008. The Mammals of China. Princeton University Press, Princeton, New Jersey.
Tandon, V. 1989. Conservation status of hog deer Cervus porcinus in India and adjacent areas. IUCN/SSC Deer Specialist Group Newsletter 7.
Than Zaw, Saw Htun, Saw Htoo Tha Po, Myint Maung, Lynam, A. J., Kyaw Thinn Latt & Duckworth, J. W. 2008. Status and distribution of small carnivores in Myanmar. Small Carnivore Conservation 38: 2?28.
Timmins, R. J. and Duckworth, J. W. 2000. Priorities for mammal conservation in the ROA: desk study for the Ecoregion-based conservation in the forests of the lower Mekong, biological assessment workshop. WWF Indochina Programme, Phnom Penh, Cambodia.
Tordoff, A. W., Timmins, R. J., Maxwell, A., Huy Keavuth, Lic Vuthy and Khou Eang Hourt (eds). 2005. Biological assessment of the Lower Mekong Dry Forests Ecoregion. pp. 192 pp.. WWF Greater Mekong Programme., Phnom Penh, Cambodia.
Whale, R. 1996. Surveying hog deer in Pakistan. Deer 10(1): 45.
Whitehead, K. G. 1993. The Whitehead Encyclopedia of Deer. Voyageur Press, Inc, Stillwater, MN, USA.
World Conservation Monitoring Centre. 1992. Status of threatened deer within protected areas: a contribution to the IUCN/SSC Action Plan. World Conservation Monitoring Centre, Cambridge, UK.
